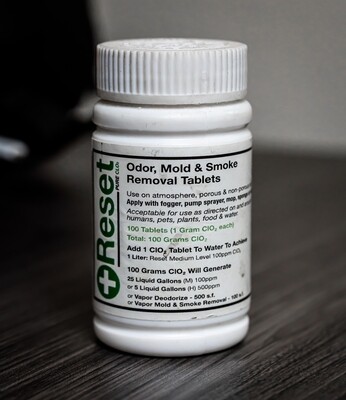
Chlorine Dioxide CLO2
"Chlorine dioxide (ClO₂) is an exceptionally effective and versatile yet low toxicity odor, mold & smoke eliminator.
ClO₂ has a 50 year history of effective use in a variety of liquid, gas & surface treatment applications including; odor, mold & smoke removal, drinking water, wastewater, bio-medical waste, medical & pharmaceutical equipment, food processing & equipment, agriculture & horticultural environments.Only ClO₂ may be generated and used as either a ‘gas vapor’ or ‘liquid’ in the atmosphere and on non-porous & porous surfaces.
Chlorine Dioxide is a chemical compound made up of one atom of chlorine and two atoms of oxygen.Do not confuse ClO₂ with typical household chlorine bleach or HTH. Chlorine dioxide is actually an oxidizing agent, not a chlorinating agent.Unlike alternative chemicals, ClO₂ reacts with organic matter through SELECTIVE OXIDATION rather than SUBSTITUTION and does not produce carcinogenic chlorinated byproducts.Only ClO₂ may be generated and used as either a ‘gas vapor’ or ‘liquid’ in the atmosphere and on non-porous & porous surfaces.
NO OTHER CHEMICAL SUBSTANCE HAS THIS CAPABILITY.
ClO₂ is a water soluble gas which penetrates the visible and non-visible barriers spraying, fogging, mops, sponges, can not. The objective of Vaporing is to release as much of the ClO₂ from the concentrate into the atmosphere as possible.Chlorine Dioxide (ClO₂) is a very small molecule 0.124 Nanometers in size. There are roughly 8 Chlorine Dioxide Gas Molecules in 1 Nanometer. 1,000 Nanometers = 1 Micron. e.g., ClO₂ vapor can penetrate into very small areas.As a gas ClO₂ will completely and evenly fill any confined space, giving it unmatched distribution and diffusion.ClO₂ has 2.5 times the oxidation capacity of Cl. Because ClO₂ is a strong oxidizer and works at significantly lower concentrations than alternative chemicals, it is significantly less corrosive.
No post ClO₂ application rinsing or wiping is required.
One of the most important qualities of chlorine dioxide is its high solubility in water, especially in cold water. Chlorine dioxide does not hydrolyze nor undergo a chemical breakdown reaction when it enters water.Instead, chlorine dioxide remains intact as a dissolved gas in water.Chlorine dioxide is approximately 10 times more soluble in water than chlorine (Cl)." - RESET Pure CLO2